Our new paper, Heitmann et al. [get PDF], is finally out! It's a collaboration between the theoretical neuroscientists Stewart Heitmann and Bard Ermentrout at the University of Pittsburgh, and the Truccolo lab at Brown University.
This work could help us understand what happens when we stimulate cerebral cortex in primates using optogenetics. Modeling how the brain responds to stimulation is important for learning how to use this new technology to control neural activity.
Optogenetic stimulation elicits gamma (~50 Hz) oscillations, the amplitude of which grows with the intensity of light stimulation. However, traveling waves away from the stimulation site also emerge.
It's difficult to reconcile oscillatory and traveling-wave dynamics in neural field models, but Heitmann et al. arrive at a surprising and testable prediction:
The observed effects can be explained by paradoxical recruitment of inhibition at low levels of stimulation, which changes cortex from a wave-propagating medium to an oscillator.
At higher stimulation levels, excitation overwhelms inhibition, giving rise to the observed gamma oscillations.
Many thanks to Stewart Heitmann, Wilson Truccolo, and Bard Ermentrout. The paper can be cited as:
Neural field simulation
Phase-triggered wave animation loops
The model predicts high-frequency (60-120 Hz) gamma oscillations within the stimulated area. It also predicts that these waves should propagate into the neighboring tissue, but at half this frequency. The result is that every-other wave in the stimulated area "sheds" a travelling wave into the areas nearby.
A re-analysis of the data recorded in Lu et al. confirms that this phenomenon is also present in vivo. The GIFs below show the waves evoked by optogenetic stimulation. These loops represent an average of the phase-locked Local Field Potential (LFP) activity in a 4×4 mm patch of primate motor cortex. On the left, we see the broadband LFP, which contains contributions from both high- and low-frequency gamma oscillations. The middle and right panels show narrow-band filtered low- and high-gamma, respectively, revealing the 2:1 mode locking.

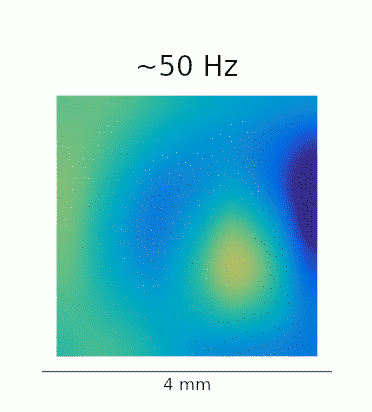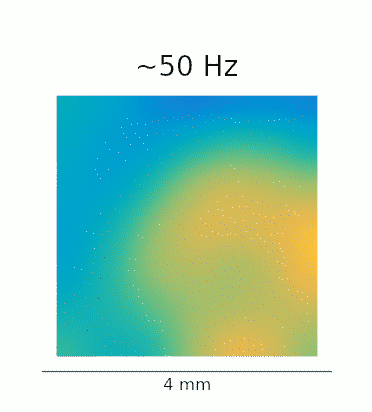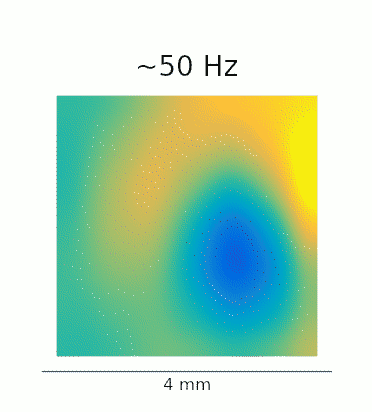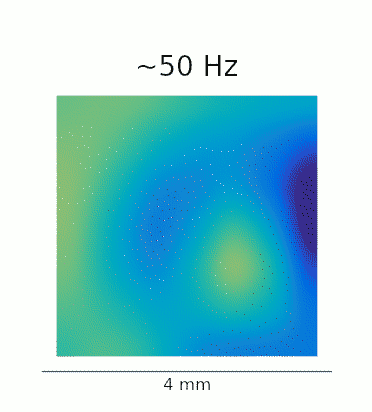

Javascript demos of the neural field model
A javascript demo of the neural field model in this paper is available on Github. Move the slider at the base to change the intensity of the optogenetic stimulation.
Supplemental figure:
Below is a summary of the LFP spectral activity evoked by optogenetic stimulation in Lu et al. It didn't make the final cut for the manuscript, so I thought i'd include it here.




No comments:
Post a Comment